by
Lauren Dubinsky, Senior Reporter | February 09, 2018
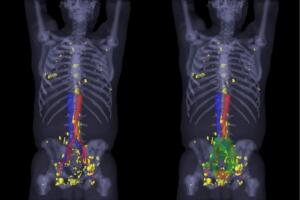
3-D map of the PSMA-positive
lesions (yellow)
Gallium-68 prostate-specific membrane antigen PET/CT imaging can spot early prostate cancer recurrence after radical prostatectomy, according to a new study published in The Journal of Nuclear Medicine.
“Currently there is no sensitive standard imaging method for spotting prostate cancer early recurrence,” Dr. Jeremie Calais of UCLA, told HCB News. “PSA is an indirect tool that reflects the burden of active prostate cancer disease in the whole body, but it cannot localize it.”
A decade after radical prostatectomy, 20 to 80 percent of patients experience prostate cancer biochemical recurrence. Salvage radiotherapy is the standard treatment option, but bone scan, CT, MR, bone PET/CT, choline PET/CT and Fluciclovine PET/CT exams are not sensitive enough to pinpoint the location of recurrence.



Ad Statistics
Times Displayed: 16169
Times Visited: 33 Final days to save an extra 10% on Imaging, Ultrasound, and Biomed parts web prices.* Unlimited use now through September 30 with code AANIV10 (*certain restrictions apply)
For the study, Calais and his team evaluated 270 patients with biochemical recurrence of prostate cancer after radical prostatectomy who were not previously treated with radiotherapy. They had each patient undergo PSMA PET/CT exams.
Almost half of the patients had a positive PSMA PET/CT and 52 had at least one PSMA-positive lesion that was not covered by the consensus clinical target volume. The team concluded that PSMA PET/CT would have a potentially significant impact on the outcomes of salvage radiotherapy for about 20 percent of the patients.
Although PSMA PET/CT is the standard of care for prostate cancer recurrence in many countries, it has not yet been approved for use in the U.S.
“Once approved and reimbursed we strongly believe that it will be rapidly implemented as standard routine care of prostate cancer patients and widely used,” said Calais.

