by
Lauren Dubinsky, Senior Reporter | July 22, 2015
Vizamyl
Courtesy of GE Healthcare
A new study presented this week at the Alzheimer’s Association International Conference revealed that GE Healthcare's Vizamyl PET amyloid imaging improved diagnostic confidence and altered care management plans for over a third of the patients with early-onset dementia.
“Thanks to the encouraging data from this study, there is growing evidence that Vizamyl can impact diagnoses and subsequent treatment decisions beyond the standard clinical workup," Ben Newton, director of neurology at GE, told HCB News. "Vizamyl could be an important resource for physicians to add to their existing workup processes when assessing early onset dementia patients.”
Vizamyl is one of three FDA approved [18F]-flutemetamol tracers, (the other two being Eli Lilly's Amyvid and Piramal Imaging's Neuraceq). It is a radiopharmaceutical used for PET imaging of beta amyloid neuritic plaque density in adults being evaluated for Alzheimer’s disease and other causes of cognitive decline.



Ad Statistics
Times Displayed: 16169
Times Visited: 33 Final days to save an extra 10% on Imaging, Ultrasound, and Biomed parts web prices.* Unlimited use now through September 30 with code AANIV10 (*certain restrictions apply)
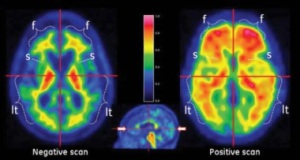
A negative scan indicates either a small amount or no plaques and reduces the likelihood that Alzheimer’s is the cause of the patient’s cognitive decline. A positive scan indicates moderate to frequent plaques, but does not confirm a diagnosis of Alzheimer’s or any other cognitive disorder.
For the study, researchers from VU University Medical Center in Amsterdam and the University of Maastricht in Netherlands used Vizamyl PET imaging to analyze 211 patients with signs of early-onset dementia — 77 percent had a pre-PET Alzheimer’s diagnosis and 33 percent had a non-Alzheimer’s diagnosis.
They found that 133 or 63 percent of the patients had positive PET scans. After physicians were informed of the PET results, 19 percent of the diagnoses were changed and overall diagnostic confidence increased from 12 to 15 percent.
The PET results also led to a change in patient management for 37 percent of the patients. Many of the patients with a positive PET scan received Alzheimer’s medication and many patients with a negative PET scan but a pre-scan Alzheimer’s diagnosis underwent ancillary investigations.
In April, the Alzheimer's Association and ACR announced a
four-year research study called The Imaging Dementia – Evidence for Amyloid Scanning (IDEAS) Study. GE is working with them to gather data, which they hope will lead to more standardized adoption of PET imaging for Alzheimer's.

