by
Lauren Dubinsky, Senior Reporter | July 18, 2014
Vizamyl
Courtesy of GE Healthcare
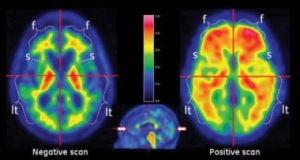
GE Healthcare presented two studies at the Alzheimer’s Association International Conference in Copenhagen on Wednesday that demonstrated the value of [18F]flutemetamol for assessing patients with cognitive disorders.
[18F]flutemetamol is GE’s investigational radiopharmaceutical product for PET imaging of beta amyloid neuritic plaque density in the brains of adults with cognitive impairment who are being evaluated for Alzheimer’s disease and other causes of cognitive impairment. It received FDA approval in October 2013 and was marketed as Vizamyl.
For the first study, the researchers injected 232 patient who had amnestic Mild Cognitive Impairment (aMCI) with [18F]flutemetamol and conducted brain scans on them. They found that the patients with positive [18F]flutemetamol scans were about 2.5 times more likely to convert to probable Alzheimer’s disease (pAD) than those with negative scans.



Ad Statistics
Times Displayed: 16169
Times Visited: 33 Final days to save an extra 10% on Imaging, Ultrasound, and Biomed parts web prices.* Unlimited use now through September 30 with code AANIV10 (*certain restrictions apply)
They concluded that [18F]flutemetamol can predict the progression from aMCI to pAD. “These findings demonstrate the potential role of [18F]flutemetamol in stratifying those patients at higher risk of developing Alzheimer’s disease, beyond its use as a diagnostic tool,” Dr. David Wolk, lead investigator of the study and assistant director of the Penn Memory Center, said in a statement.
For the second study, 80 patients with early onset dementia under 70 years of age and with physician diagnostic confidence under 90 percent, received [18F]flutemetamol PET scans that were then categorized as either amyloid positive or negative.
The researchers found that using [18F]flutemetamol led to a higher rate of diagnostic confidence for physicians and helped to verify or rule out an Alzheimer’s disease diagnosis in many of the patients. More specifically, their confidence rose from 67 percent to 90 percent.
After a review of the [18F]flutemetamol scan, 20 percent of the patients had a change in diagnosis. The clinical diagnosis changed in 12 of the 15 patients who were diagnosed with Alzheimer’s disease before the [18F]flutemetamol scan and who had an amyloid negative result.
Additionally, 48 percent of the patients had a change in patient health care management after the results of the [18F]flutemetamol PET results.
“Early and accurate diagnoses may have implications for both prognosis and treatment among patients with early onset dementia,” Dr. Marissa Zwan, of the VU University Medical Center and lead investigator of the study, said in a statement. “Greater diagnostic confidence supports better patient management and helps physicians to determine appropriate treatment options, as well as helping patients and caregivers to plan for the future.”
Back to HCB News

