by
Loren Bonner, DOTmed News Online Editor | October 25, 2013
GE Healthcare announced today that its PET imaging agent for Alzheimer's has been approved for market by the U.S. Food and Drug Administration.
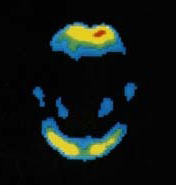
Vizamyl (flutemetamol F 18 injection) is used to visually detect beta amyloid plaque, which is associated with Alzheimer's and dementia, in the brains of patients being evaluated for the conditions.
It's now the second brain imaging drug for Alzheimer's disease and dementia on the market. Eli Lilly & Co.'s Amyvid was the first. It received FDA approval in the spring of 2012.



Ad Statistics
Times Displayed: 42287
Times Visited: 1164 Ampronix, a Top Master Distributor for Sony Medical, provides Sales, Service & Exchanges for Sony Surgical Displays, Printers, & More. Rely on Us for Expert Support Tailored to Your Needs. Email info@ampronix.com or Call 949-273-8000 for Premier Pricing.
According to FDA language, Vizamyl works by attaching to beta amyloid and producing a PET image of the brain that is used to evaluate the plaque. A negative Vizamyl scan means that there is little or no beta amyloid accumulation in the brain and the cause of the dementia is probably not due to Alzheimer's. A positive scan means that there is probably a moderate or greater amount of amyloid in the brain, but it does not establish a diagnosis of Alzheimer's or other dementia.
"Many Americans are evaluated every year to determine the cause of diminishing neurologic functions, such as memory and judgment, that raise the possibility of Alzheimer's disease," said Dr. Shaw Chen, from the FDA's Center for Drug Evaluation and Research, in a statement. "Imaging drugs like Vizamyl provide physicians with important tools to help evaluate patients for AD and dementia."
In July 2012, GE released
results from a phase 3 study on flutemetamol, which formed the basis of the FDA
application in January 2013. In that study, flutemetamol was able to successfully measure beta-amyloid plaque in living patients.
Vizamyl is manufactured for GE Healthcare by Medi-Physics, Inc., based in Arlington Heights, Ill.