by
Loren Bonner, DOTmed News Online Editor | October 16, 2013
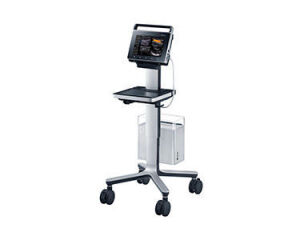
Samsung UGEO PT60A
tablet-based ultrasound
The medical device industry is ready to go mobile and Samsung Electronics America Inc. wants to take ultrasound along for the ride.
The company announced the release of its first tablet-based ultrasound system, the UGEO PT60A. The system received 510(k) U.S. Food and Drug Administration clearance in August and completed its domestic product review in September.
"The Samsung UGEO PT60A was developed specifically for the point-of-care market, which is the fastest growing market in the ultrasound modality," Jason Head, senior manager for point-of-care ultrasound at the health and medical equipment unit of Samsung Electronics America, told DOTmed News.
Head said that ultrasound is becoming a standard of care for many applications in the medical field for both diagnostics and procedural care, including needle guidance. He said ultrasound is also attractive because it can help reduce some of the concerns health care providers have around radiation exposure.
Samsung's push into the medical device space came in 2010 with the acquisition of Medison Co., a maker of ultrasound equipment. Ever since then, they have been releasing new ultrasound systems to the market,
including two earlier this month.
But Samsung is not the first out of the gate with a tablet-based ultrasound system. For example, the medical device startup Mobisante introduced one in April, two years after the release of its smartphone-based ultrasound system. Mobisante's CTO told MobihealthNews back in April that tablet-based systems provide a good compromise between the portability of a smartphone ultrasound and the high screen resolution of a traditional ultrasound machine.
Samsung is also touting superb image quality on the UGEO PT60A with SDMR technology, which Head said provides clearer imaging with a new noise reduction filter.
Samsung showcased its latest product at this week's American Society of Anesthesiology's annual meeting in San Francisco.
