by
Olga Deshchenko, DOTmed News Reporter | March 30, 2011
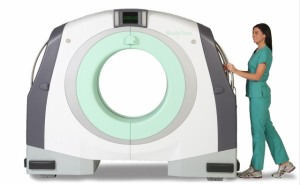
Neurologica Corp. said Wednesday it received U.S. Food and Drug Administration approval for its BodyTom, a pioneering portable, full body multi-slice CT scanner.
The scanner has an 85-centimeter gantry and a 60-centimeter field of view. Similar to a portable chest X-ray system, the BodyTom can be moved from room to room and used in a variety of settings, including intensive care units, emergency departments and operating rooms, according to the company.
[Read about the growing trend of CT in the ER and OR]



Ad Statistics
Times Displayed: 16169
Times Visited: 33 Final days to save an extra 10% on Imaging, Ultrasound, and Biomed parts web prices.* Unlimited use now through September 30 with code AANIV10 (*certain restrictions apply)
"Our goal with the BodyTom is to deliver high quality CT imaging in a completely portable package that is capable of scanning an entire patient's body," Dr. Colin McDonald, chief medical officer of NeuroLogica, said in prepared remarks.
The Danvers, Mass.-based company also manufactures the CereTom, a small bore, 8-slice portable CT scanner for brain perfusion and brain angiography.
The BodyTom is commercially available this year, the company said.