*Rx Only - We do not sell or ship to the state of California
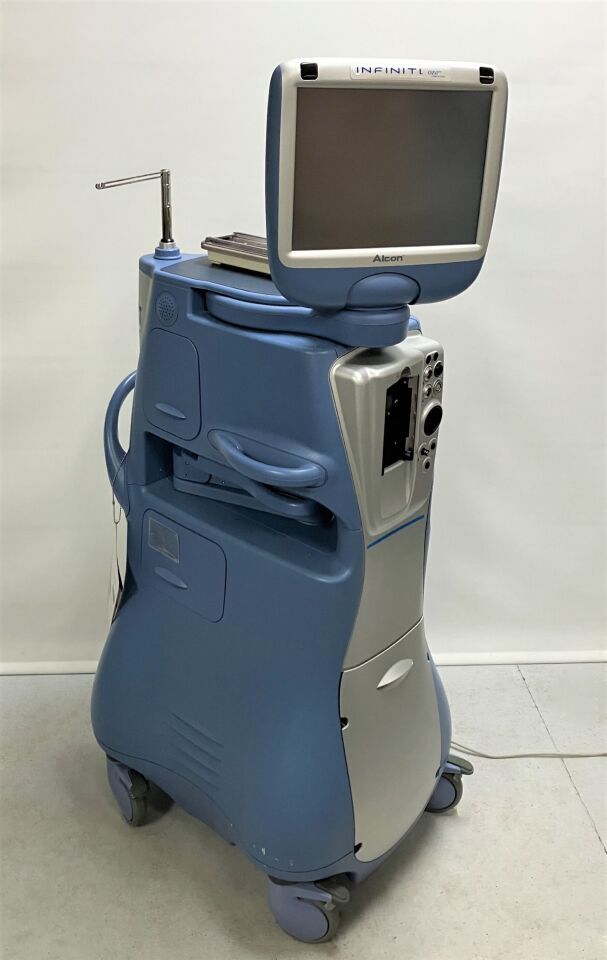
Alcon Infiniti Vision System:
*Ref: 210-0000-503
*MFG: 2008-01
*Made in USA
*Ozil Compatible
*Movable Instrument Tray
*Two Accessory Drawers
*One Footswitch Drawer
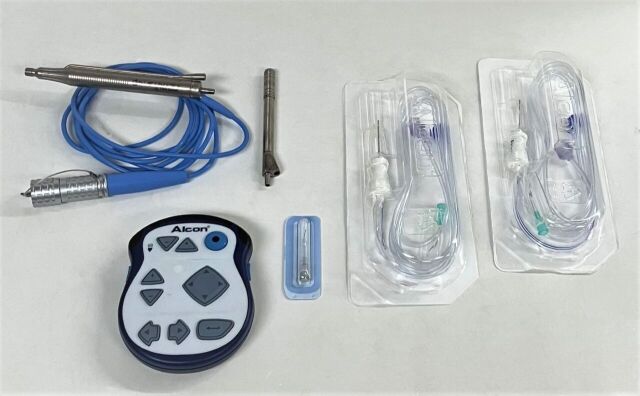
Alcon Infiniti Ultrasonic Handpiece:
*Ref: 8065750121
*Phacoemulsifier Handpiece
*Tips: 110403, 062603
*Sleeves: Light Purple 0.9 mm High Infusion Sleeves (Qty: 3), Light Blue 1.1 mm High Infusion Sleeves (Qty: 3)
*Extra Handpiece: 016417
Also Includes:
*Alcon Infiniti 8065750403 Enhanced Footswitch, MFG: 2005-12
*Alcon Infiniti 00380657504688 Remote Control, MFG: 2016-12-02, Made in USA, Powered by 3 AAA/LR03 Batteries
*Alcon Infiniti 8065750157 Anterior Vitrectomy Probe Pack with 21GA Infusion Cannula, MFG: 2019-01-16, EXP: 2021-12-31, Single Use, New, Qty: 2
*27G Anterior Chamber Cannula
*Reference Guide
The sale of this item may be subject to regulation by the U.S. Food and Drug Administration and state and local regulatory agencies. If so, do not bid on this item unless you are an authorized purchaser. If the item is subject to FDA regulation, I will verify your status as an authorized purchaser of this item before shipping of the item." This medical device has been used and has not been altered. The item will be properly cleaned and sterilized (where required) before shipment." BY PURCHASING YOU ACKNOWLEDGE TO BE AN AUTHORIZED BUYER.
FDA REGULATIONS: The sale of this item may be subject to regulation by the U.S. Food and Drug Administration and state and local regulatory agencies. If so, do not offer on this item this item unless you are an authorized purchaser. If the item is subject to FDA regulation, I will verify your status as an authorized purchaser of this item before shipping of the item. If you have questions about legal obligations regarding sales of medical devices, you should consult with the FDA's Center for Devices and Radiological Health:
Links: http://www.fda.gov/cdrh/devadvice/ or number: 800.638.2041email: dsmica@cdrh.fda.gov
Thanks for your cooperation!
DISCLAIMER: Regardless of the origin of the equipment, documentation provided or identification appearing upon the equipment, the equipment described and offered here is in no way certified for, recommended for, or offered for any specific use. The purchaser agrees that the seller shall not be held responsible or liable for any injuries or damages, whether incidental or consequential, associated in any way with the equipment. The purchaser, by offering on this equipment, indicates their acknowledgment of, and agreement to the terms of this disclaimer.
Return Policy
Items are sold as-is with no returns or refunds available unless explicitly stated.