Breast cancer screening gets personal
July 19, 2021
by Lisa Chamoff, Contributing Reporter
The COVID-19 pandemic continues to impact the healthcare market and the breast screening space in particular. Equipment and software manufacturers are focusing on improvements to existing products or working on solutions to help their customers handle screening backlogs.
Companies are also developing technologies to help increase workflow and, in the case of one software maker, looking to breast imaging to provide insight into another deadly and costly healthcare problem: heart disease.
Here’s a look at what’s new in the last year from more than a dozen companies.
Canon Medical Systems
At the end of 2019, Canon Medical Systems introduced 24-megahertz transducers for the Aplio i700 and i800 ultrasound systems and a new 18-megahertz transducer for the Aplio a550 and a450 systems.
Last year, the company introduced FleXstyle, which enables easy sonographer adaptation and operational flexibility, with a programmable touch screen panel for tailored layout and electric lifting for easy up and down of the control panel. FleXstyle is available for all ultrasound imaging and allows for an intuitive workflow and better ergonomics for sonographers.
“Basically, the system becomes a chameleon and will work for you,” said Tina Hodgson, senior manager of solutions marketing for ultrasound at Canon Medical Systems.
If a facility is transferring from another system to Canon, the hard keys can be changed.
“Muscle memory is a great thing,” Hodgson said. “If you’re used to having the button there, we can put the button there.”
CureMetrix
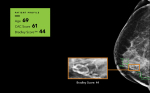
CureMetrix is planning to seek FDA clearance for a new product that can assess a woman’s risk of heart disease from the same mammogram used to identify breast cancer. The product, called cmAngio, looks for arterial calcifications, normally disregarded on a mammogram, that may show evidence of peripheral vascular disease.
Studies show breast arterial calcification is an indicator of cardiovascular disease. cmAngio calculates a Bradley score for the mammography report and a letter goes to the referring physician noting the patient’s risk of developing heart disease, the leading cause of death for women in the United States.
“What a tool like this represents is the potential to screen 40 million women a year for heart disease in the U.S. alone,” said Kevin Harris, the president of CureMetrix, who estimates a $30 billion healthcare economic impact over five years.
The company is signing research agreements with institutions across the country and is aiming to have the product FDA-cleared by the end of the year.
Densitas
This year, Densitas updated its densitas intelliMammo A.I. Platform. The product brings together the company’s qualityAI, densityAI and riskAI solutions, and was expanded with a novel solution to simplify compliance with MQSA EQUIP criteria. It shows image quality metrics by technologist, and by machine and site, many different factors, providing reports and corrective action documentation. The image quality metrics also enable mammography educators to develop customized evidence-based training curriculums.
“We have worked with mammography educators to provide image quality metrics and positioning tips at the technologist’s fingertips,” said Mo Abdolell, chief executive officer of Densitas.
FUJIFILM Medical Systems U.S.A., Inc.
This past year, Fujifilm introduced a new 18-centimeter-by-24-centimeter tomo spot paddle with 10-centimeter round compression for the ASPIRE Cristalle digital mammography system. This paddle targets a suspicious area for compression and allows for tissue visualization outside of the round spot area, keeping the metal frame completely outside of the image.
The panel was developed based on customer feedback, said Christine Murray, women’s health project manager for FUJIFILM Medical Systems U.S.A., Inc.
“With other paddles, the spot is the only thing you’re going to be imaging,” Murray said. “Our new paddle allows some additional compression outside the area of focus.”
The company also expanded its partnership with Volpara Health and is using its VolparaLive! and Volpara Enterprise.
GE Healthcare
Last year, GE Healthcare introduced Serena Bright, the industry’s first contrast-enhanced guided biopsy solution. The technology allows clinicians to conduct breast biopsy exams with contrast guidance using the same mammography equipment, in the same room and with the same staff as the screening or diagnostic mammogram, which can be done faster and at a lower cost than an MR-guided biopsy.
Key to the technology is GE Healthcare's SenoBright HD Contrast Enhanced Spectral Mammography (CESM), which highlights areas of unusual blood flow to help localize lesions that need to be biopsied.
“We’re very excited to bring this industry-first device to the market,” said Agnes Berzsenyi, president and chief executive officer of women’s health for GE Healthcare
Also last year, the company announced a partnership with the American College of Radiology and the Breast Cancer Research Foundation for a contrast-enhanced mammography trial. The paired-design multicenter trial seeks to determine if contrast-enhanced spectral mammography screening provides more accurate cancer detection compared to the combination of digital breast tomosynthesis and whole breast ultrasound in women with dense breasts, who are ages 40 to 75 and at average-to-intermediate risk for breast cancer. The trial is expected to launch soon.
Last year, the company launched a global Don’t Skip campaign, centered around sharing best practices among customers and urging patients via social media to keep up with regular breast cancer screening.
“We’re all about making a difference and early detection is the best defense against breast cancer,” Berzsenyi said.
Hologic
In October of last year, Hologic launched a multiyear initiative to increase breast cancer screening for Black women. In May, the company announced Project Health Equality, designed to reduce healthcare disparities. It also initiated a Back To Screening campaign in August, encouraging women to prioritize annual mammograms delayed due to the COVID-19 pandemic
The company also continued to build on its recent acquisition of ultrasound company Supersonic Imagine. In July 2020 it launched the premium SuperSonic MACH 40, which features a greater frame rate of 20,000 acquisitions per second and ShearWave PLUS elastography. In December 2020 it introduced 3D imaging for MACH 40.
“This increases diagnostic confidence and can envision dense breast better,” said Samir Parikh, global vice president of research and development and clinical affairs for Hologic.
Pairing 3D imaging with elastography can also accurately measure tumor size and ensure clear margin definition in preoperative settings, Parikh said.
In January 2021, the company announced the MACH 20 for general and specialty imaging, including breast.
In the screening space, December 2020, the company received FDA clearance for its Genius AI Detection technology, a new deep learning-based software designed to help radiologists detect subtle potential cancers in breast tomosynthesis images.
“This triage and decision support software focuses on how we categorize and prioritize cases based on complexity,” Parikh said.
There were also advances in the biopsy and surgery space. Last August, Hologic announced improvements for its Brevera Breast Biopsy solution, which allows providers to do vacuum-assisted tissue acquisition with real-time imaging verification and advanced post-biopsy handling in one, integrated system.
This “simplifies handling while maintaining the core integrity,” with a disposable needle and reusable device driver
“You can sterilize the driver to simplify storage and improve waste management,” Parikh said.
In November 2020, Hologic launched its PERL Ring Localization Device. Placed up to a few days before breast surgery, it provides a suture-like flexible tail without the drawbacks of traditional stiff wire-localizations. It provides real-time visual and tactile feedback designed to minimize the risk of migration, Parikh said.
After acquiring Faxitron Bioptics in 2018, Hologic introduced the Faxitron CT Specimen Radiography System to the marketplace in January 2020. This 3D CT system is designed to help achieve better surgical outcomes and improve intraoperative specimen margin assessment by providing data from all three axes, beyond tomosynthesis. During 3D reconstruction, the automatic metal artifact correction minimizes interference from metal clips and markers.
iCAD
In March, iCAD received FDA approval for ProFound AI Version 3.0 for digital breast tomosynthesis (DBT). The new version of the software offers up to a 10% improvement in specificity and up to 40% faster processing times compared to previous versions of the software.
In the second half of 2020, the company released ProFound AI Risk for 2D mammography. It plans to release the solution for DBT next quarter.
The software looks at three features — age, breast density and subtle mammographic features, — to provide an “accurate two-year risk assessment that is truly personalized for each woman,” said Stacey Stevens, president of iCAD.
“Instead of recommending that every woman who turns 40 be screened with mammography we believe the future of screening will be based on individual risk,” Stevens said. “This technology is going to make mammography screening more personalized, and we are proud to be on the leading-edge of this exciting paradigm shift that will truly have a positive impact on patient care.”
Ikonopedia
Ikonopedia recently launched a new product called Ikonopedia Insight, an interactive data visualization tool that provides insight into patient and clinical data already entered into the system, including patient information, imaging exams and follow-up, to generate a variety of BI-RADS-compliant static reports that give a high-level summary of key information.
“Taking the idea of making data work for customers now goes a step further,” said Emily Crane, Ikonopedia's chief executive officer.
Through interactive, colorful and frequently-updated visualizations, customers will be able to learn more about patient demographics and determine who may need more frequent screenings, identify organizational operational trends to decide where staffing adjustments may be needed to address patient volume load, identify personnel and potential training gaps and more.
“The solution uses Tableau reporting tools to create end-to-end reports,” Crane said. “For example, facilities can research breast screening based on patient demographics and risk.”
“Before, we could create reports for clients, but they couldn’t do it on their own,” Crane said. “Clients are making actionable changes to how they’re doing things based on the reports, including implementing improvements in high-risk follow-up, resolution of aging BI-RADS and even patient education across specific demographics.”
Additionally, in January, Ikonopedia added questions to its survey to help identify and track the impact COVID-19 has had on breast imaging and to help aid doctors in identifying patients who had recently had the COVID-19 vaccine, so they weren’t performing unnecessary biopsies.
“After worrying patients would delay screening, providers can find out how many cancers were actually delayed in being diagnosed,” Crane said.
Koning Corporation
Koning continues to market its Koning Breast CT (KBCT) system. On January 1, six new Current Procedural Terminology (CPT) codes were put into use after approval by the American Medical Association, providing the first step toward reimbursement for breast CT in place of a diagnostic mammographic workup.
Facilities now use the system after the screening mammogram when diagnostic workup is required. Prior to dedicated codes, facilities were required to use unlisted codes, making reimbursement uncertain.
“We are getting a significant uptick in use and greater reimbursement per patient,” said David Georges, president of the company’s USA division. “We anticipate that the option of breast CT will ramp up.”
The company is performing a study in advance of seeking FDA approval to use the system for breast cancer screening.
Medical Scientific
Medical Scientific recently received a CE Mark for its new line of Harmonia digital mammography systems. The product line includes three models: an entry-level 2D system, a 2D system that’s upgradable for digital breast tomosynthesis and a digital breast tomosynthesis system.
The new systems feature an improved detector with a large dynamic range and improved image acquisition software.
The detector has the highest resolution for mammography with a small pixel size of 49.5 microns, said Steven Veyland, chief operating officer of Medical Scientific, allowing it to resolve smaller details at an earlier stage.
“We were also able to develop a new way of image acquisition,” Veyland said. “It captures images at different dynamic range for enhanced imaging of both 2D and Tomosynthesis.”
Micrima
Micrima, which manufactures a breast scanning system that uses radio-waves instead of X-rays, is submitting its seventh-generation device for CE approval.
The latest version of the device has better sensitivity and is less reliant on expensive components, said Adrian Waller, the chief executive officer of Micrima.
The device, which Waller says shows high sensitivity for dense breast tissue, is currently used as a radiation-free adjunctive tool in the European market. The company’s goal in the next six to 12 months is to develop classifiers that will allow it to be used as a primary screening tool.
“Our mission is to help healthcare professionals save lives by detecting cancer earlier. We believe the best way to do this is to replace mammography in screening,” Waller said.
PenRad
In August, PenRad, which provides mammography tracking, reporting and compliance software, launched PenExpress, which allows patients to update their personal health history and complete high-risk and genetic testing eligibility documents from their home, prior to their appointment. Working in concert with PenConnect and PenForms, the patient gets a secure link and can complete the documentation, avoiding paper forms and crowded waiting rooms.
“PenRad clients report patients appreciate the opportunity to spend less time in waiting rooms, more time to do a thorough job of completing their risk history forms, and with PenConnect, receiving their exam result electronically the same day as their appointment,” said Daniel Bickford, marketing director for PenRad.
PenRad also recently partnered with Myriad Genetics to offer a high-risk and genetics screening package and streamlined patient access to Myriad’s genetic counselors for participating clinics.
Philips
This year, Philips updated its Ultimate Breast Solution by introducing Microflow Imaging High Definition (MFI HD), which provides twice the sensitivity and twice the resolution compared to legacy MFI, according to the company. Additionally, contrast imaging has been improved for breast application. These enhancements will extend the integrated breast solution using the Anatomical Intelligent Breast scanning assistant from Philips, which combines advanced imaging, full solution elastography and precision biopsy.
The solution, designed for diagnosis, screening and follow-up in the case of suspected lesions, is available on EPIQ and Affiniti ultrasound systems. Recently, the monitor size has also been increased to 24 inches with the HD MAX monitor, said Radjen Ganpat, marketing manager for Ultrasound at Philips.
Current customers can upgrade to the latest enhancements.
Qlarity Imaging
Qlarity Imaging is moving its QuantX AI-based decision support software, for helping radiologists in the assessment and characterization of breast abnormalities during breast MR exams, from a traditional dedicated workstation to a vendor-neutral, cloud-based format. This will allow radiologists to use their own viewer and leverage their existing PACS environment, access the secure application anywhere and automatically export their findings into the reporting software.
“We reimagined what breast radiologists need from their technology tools,” said Jon T. DeVries, the chief executive officer of Qlarity Imaging. “With this new format, our goal is to address many of the current challenges health systems and radiologists face and encourage scalable technology that strengthens our vision of transforming the patient journey, including new developments in screening procedures.”
The company plans to submit the new release to the FDA this month and is aiming for clearance in the fourth quarter of 2021.
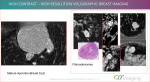
QT Imaging
QT Imaging has developed a technology called Quantitative Transmission (QT) ultrasound, which uses transmitted sound waves to create three dimensional images of the breast. The technology is different from reflection ultrasound, using 2048 cameras and a water bath to image the breast without compression.
The technology is currently used for both regular and adjunctive screening, and the company is looking to eventually provide an alternative to screening mammography, said John Klock, chief executive officer and chief medical officer of QT Imaging.
A blinded clinical trial comparing the technology to mammography was published in Academic Radiology online in December 2020. The study found that QT scan provided better sensitivity and specificity than mammography, resulting in fewer callbacks.
“We're very happy that women are getting good results from it,” Klock said. “We're hoping women will be our allies.”
The company plans to expand the indications for use and expects to submit for FDA clearance to measure fibroglandular volume in 2021, which can be affected by a woman’s menstrual cycle or hormone therapy.
“For women on hormone therapy to prevent breast cancer, this will allow measurement of how they’re responding,” Klock said.
Siemens Healthineers
Siemens Healthineers has not debuted any new women's health systems or software within the last year. However, the company has been strategizing with its customers to help them cope with the sudden influx of screening examinations following the screening mammography standstill brought on by the pandemic.
For example, the latest software update on MAMMOMAT Revelation has decreased room time, providing a four-view tomosynthesis exam in under six minutes, allowing facilities to provide 10-minute exam slots.
“Our customers were really valuing that coming out of the pandemic,” said Abigail Weldon, director of women’s health at Siemens Healthineers North America.
Automated breast density reading helps radiologists prioritize further diagnostic needs right away.
The company also offers remote installation, training and support.
Volpara
In January, Volpara Health acquired CRA Health, which has an expertise in breast cancer risk and genetics. This best-of-breed breast cancer risk assessment solution was integrated into Volpara’s Patient Hub mammography reporting and patient communication software. The new solution also provides content to help radiologists explain the patient’s breast cancer risk alongside appropriate recommendations, including whether additional imaging or genetics testing is needed according to established guidelines.
“It’s all about personalizing breast care and helping save families from cancer by preventing advanced stage breast cancer,” said Ralph Highnam, chief executive officer of Volpara Health.
Last fall, the company also launched Project Thumb, a new tool that will allow clinicians to embed mammogram images on patients' breast density notification letters with the goal to educate patients to help them make informed decisions about their breast care.
Later this year, the company plans to upgrade its Volpara Analytics platform, which enables technologists to log in and learn about their own acquisition performance and access education on quality imaging techniques.
Companies are also developing technologies to help increase workflow and, in the case of one software maker, looking to breast imaging to provide insight into another deadly and costly healthcare problem: heart disease.
Here’s a look at what’s new in the last year from more than a dozen companies.
Canon Medical Systems
At the end of 2019, Canon Medical Systems introduced 24-megahertz transducers for the Aplio i700 and i800 ultrasound systems and a new 18-megahertz transducer for the Aplio a550 and a450 systems.
Last year, the company introduced FleXstyle, which enables easy sonographer adaptation and operational flexibility, with a programmable touch screen panel for tailored layout and electric lifting for easy up and down of the control panel. FleXstyle is available for all ultrasound imaging and allows for an intuitive workflow and better ergonomics for sonographers.
“Basically, the system becomes a chameleon and will work for you,” said Tina Hodgson, senior manager of solutions marketing for ultrasound at Canon Medical Systems.
If a facility is transferring from another system to Canon, the hard keys can be changed.
“Muscle memory is a great thing,” Hodgson said. “If you’re used to having the button there, we can put the button there.”
CureMetrix
CureMetrix is planning to seek FDA clearance for a new product that can assess a woman’s risk of heart disease from the same mammogram used to identify breast cancer. The product, called cmAngio, looks for arterial calcifications, normally disregarded on a mammogram, that may show evidence of peripheral vascular disease.
Studies show breast arterial calcification is an indicator of cardiovascular disease. cmAngio calculates a Bradley score for the mammography report and a letter goes to the referring physician noting the patient’s risk of developing heart disease, the leading cause of death for women in the United States.
“What a tool like this represents is the potential to screen 40 million women a year for heart disease in the U.S. alone,” said Kevin Harris, the president of CureMetrix, who estimates a $30 billion healthcare economic impact over five years.
The company is signing research agreements with institutions across the country and is aiming to have the product FDA-cleared by the end of the year.
Densitas
This year, Densitas updated its densitas intelliMammo A.I. Platform. The product brings together the company’s qualityAI, densityAI and riskAI solutions, and was expanded with a novel solution to simplify compliance with MQSA EQUIP criteria. It shows image quality metrics by technologist, and by machine and site, many different factors, providing reports and corrective action documentation. The image quality metrics also enable mammography educators to develop customized evidence-based training curriculums.
“We have worked with mammography educators to provide image quality metrics and positioning tips at the technologist’s fingertips,” said Mo Abdolell, chief executive officer of Densitas.
FUJIFILM Medical Systems U.S.A., Inc.
This past year, Fujifilm introduced a new 18-centimeter-by-24-centimeter tomo spot paddle with 10-centimeter round compression for the ASPIRE Cristalle digital mammography system. This paddle targets a suspicious area for compression and allows for tissue visualization outside of the round spot area, keeping the metal frame completely outside of the image.
The panel was developed based on customer feedback, said Christine Murray, women’s health project manager for FUJIFILM Medical Systems U.S.A., Inc.
“With other paddles, the spot is the only thing you’re going to be imaging,” Murray said. “Our new paddle allows some additional compression outside the area of focus.”
The company also expanded its partnership with Volpara Health and is using its VolparaLive! and Volpara Enterprise.
GE Healthcare
Last year, GE Healthcare introduced Serena Bright, the industry’s first contrast-enhanced guided biopsy solution. The technology allows clinicians to conduct breast biopsy exams with contrast guidance using the same mammography equipment, in the same room and with the same staff as the screening or diagnostic mammogram, which can be done faster and at a lower cost than an MR-guided biopsy.
Key to the technology is GE Healthcare's SenoBright HD Contrast Enhanced Spectral Mammography (CESM), which highlights areas of unusual blood flow to help localize lesions that need to be biopsied.
“We’re very excited to bring this industry-first device to the market,” said Agnes Berzsenyi, president and chief executive officer of women’s health for GE Healthcare
Also last year, the company announced a partnership with the American College of Radiology and the Breast Cancer Research Foundation for a contrast-enhanced mammography trial. The paired-design multicenter trial seeks to determine if contrast-enhanced spectral mammography screening provides more accurate cancer detection compared to the combination of digital breast tomosynthesis and whole breast ultrasound in women with dense breasts, who are ages 40 to 75 and at average-to-intermediate risk for breast cancer. The trial is expected to launch soon.
Last year, the company launched a global Don’t Skip campaign, centered around sharing best practices among customers and urging patients via social media to keep up with regular breast cancer screening.
“We’re all about making a difference and early detection is the best defense against breast cancer,” Berzsenyi said.
Hologic
In October of last year, Hologic launched a multiyear initiative to increase breast cancer screening for Black women. In May, the company announced Project Health Equality, designed to reduce healthcare disparities. It also initiated a Back To Screening campaign in August, encouraging women to prioritize annual mammograms delayed due to the COVID-19 pandemic
The company also continued to build on its recent acquisition of ultrasound company Supersonic Imagine. In July 2020 it launched the premium SuperSonic MACH 40, which features a greater frame rate of 20,000 acquisitions per second and ShearWave PLUS elastography. In December 2020 it introduced 3D imaging for MACH 40.
“This increases diagnostic confidence and can envision dense breast better,” said Samir Parikh, global vice president of research and development and clinical affairs for Hologic.
Pairing 3D imaging with elastography can also accurately measure tumor size and ensure clear margin definition in preoperative settings, Parikh said.
In January 2021, the company announced the MACH 20 for general and specialty imaging, including breast.
In the screening space, December 2020, the company received FDA clearance for its Genius AI Detection technology, a new deep learning-based software designed to help radiologists detect subtle potential cancers in breast tomosynthesis images.
“This triage and decision support software focuses on how we categorize and prioritize cases based on complexity,” Parikh said.
There were also advances in the biopsy and surgery space. Last August, Hologic announced improvements for its Brevera Breast Biopsy solution, which allows providers to do vacuum-assisted tissue acquisition with real-time imaging verification and advanced post-biopsy handling in one, integrated system.
This “simplifies handling while maintaining the core integrity,” with a disposable needle and reusable device driver
“You can sterilize the driver to simplify storage and improve waste management,” Parikh said.
In November 2020, Hologic launched its PERL Ring Localization Device. Placed up to a few days before breast surgery, it provides a suture-like flexible tail without the drawbacks of traditional stiff wire-localizations. It provides real-time visual and tactile feedback designed to minimize the risk of migration, Parikh said.
After acquiring Faxitron Bioptics in 2018, Hologic introduced the Faxitron CT Specimen Radiography System to the marketplace in January 2020. This 3D CT system is designed to help achieve better surgical outcomes and improve intraoperative specimen margin assessment by providing data from all three axes, beyond tomosynthesis. During 3D reconstruction, the automatic metal artifact correction minimizes interference from metal clips and markers.
iCAD
In March, iCAD received FDA approval for ProFound AI Version 3.0 for digital breast tomosynthesis (DBT). The new version of the software offers up to a 10% improvement in specificity and up to 40% faster processing times compared to previous versions of the software.
In the second half of 2020, the company released ProFound AI Risk for 2D mammography. It plans to release the solution for DBT next quarter.
The software looks at three features — age, breast density and subtle mammographic features, — to provide an “accurate two-year risk assessment that is truly personalized for each woman,” said Stacey Stevens, president of iCAD.
“Instead of recommending that every woman who turns 40 be screened with mammography we believe the future of screening will be based on individual risk,” Stevens said. “This technology is going to make mammography screening more personalized, and we are proud to be on the leading-edge of this exciting paradigm shift that will truly have a positive impact on patient care.”
Ikonopedia
Ikonopedia recently launched a new product called Ikonopedia Insight, an interactive data visualization tool that provides insight into patient and clinical data already entered into the system, including patient information, imaging exams and follow-up, to generate a variety of BI-RADS-compliant static reports that give a high-level summary of key information.
“Taking the idea of making data work for customers now goes a step further,” said Emily Crane, Ikonopedia's chief executive officer.
Through interactive, colorful and frequently-updated visualizations, customers will be able to learn more about patient demographics and determine who may need more frequent screenings, identify organizational operational trends to decide where staffing adjustments may be needed to address patient volume load, identify personnel and potential training gaps and more.
“The solution uses Tableau reporting tools to create end-to-end reports,” Crane said. “For example, facilities can research breast screening based on patient demographics and risk.”
“Before, we could create reports for clients, but they couldn’t do it on their own,” Crane said. “Clients are making actionable changes to how they’re doing things based on the reports, including implementing improvements in high-risk follow-up, resolution of aging BI-RADS and even patient education across specific demographics.”
Additionally, in January, Ikonopedia added questions to its survey to help identify and track the impact COVID-19 has had on breast imaging and to help aid doctors in identifying patients who had recently had the COVID-19 vaccine, so they weren’t performing unnecessary biopsies.
“After worrying patients would delay screening, providers can find out how many cancers were actually delayed in being diagnosed,” Crane said.
Koning Corporation
Koning continues to market its Koning Breast CT (KBCT) system. On January 1, six new Current Procedural Terminology (CPT) codes were put into use after approval by the American Medical Association, providing the first step toward reimbursement for breast CT in place of a diagnostic mammographic workup.
Facilities now use the system after the screening mammogram when diagnostic workup is required. Prior to dedicated codes, facilities were required to use unlisted codes, making reimbursement uncertain.
“We are getting a significant uptick in use and greater reimbursement per patient,” said David Georges, president of the company’s USA division. “We anticipate that the option of breast CT will ramp up.”
The company is performing a study in advance of seeking FDA approval to use the system for breast cancer screening.
Medical Scientific
Medical Scientific recently received a CE Mark for its new line of Harmonia digital mammography systems. The product line includes three models: an entry-level 2D system, a 2D system that’s upgradable for digital breast tomosynthesis and a digital breast tomosynthesis system.
The new systems feature an improved detector with a large dynamic range and improved image acquisition software.
The detector has the highest resolution for mammography with a small pixel size of 49.5 microns, said Steven Veyland, chief operating officer of Medical Scientific, allowing it to resolve smaller details at an earlier stage.
“We were also able to develop a new way of image acquisition,” Veyland said. “It captures images at different dynamic range for enhanced imaging of both 2D and Tomosynthesis.”
Micrima
Micrima, which manufactures a breast scanning system that uses radio-waves instead of X-rays, is submitting its seventh-generation device for CE approval.
The latest version of the device has better sensitivity and is less reliant on expensive components, said Adrian Waller, the chief executive officer of Micrima.
The device, which Waller says shows high sensitivity for dense breast tissue, is currently used as a radiation-free adjunctive tool in the European market. The company’s goal in the next six to 12 months is to develop classifiers that will allow it to be used as a primary screening tool.
“Our mission is to help healthcare professionals save lives by detecting cancer earlier. We believe the best way to do this is to replace mammography in screening,” Waller said.
PenRad
In August, PenRad, which provides mammography tracking, reporting and compliance software, launched PenExpress, which allows patients to update their personal health history and complete high-risk and genetic testing eligibility documents from their home, prior to their appointment. Working in concert with PenConnect and PenForms, the patient gets a secure link and can complete the documentation, avoiding paper forms and crowded waiting rooms.
“PenRad clients report patients appreciate the opportunity to spend less time in waiting rooms, more time to do a thorough job of completing their risk history forms, and with PenConnect, receiving their exam result electronically the same day as their appointment,” said Daniel Bickford, marketing director for PenRad.
PenRad also recently partnered with Myriad Genetics to offer a high-risk and genetics screening package and streamlined patient access to Myriad’s genetic counselors for participating clinics.
Philips
This year, Philips updated its Ultimate Breast Solution by introducing Microflow Imaging High Definition (MFI HD), which provides twice the sensitivity and twice the resolution compared to legacy MFI, according to the company. Additionally, contrast imaging has been improved for breast application. These enhancements will extend the integrated breast solution using the Anatomical Intelligent Breast scanning assistant from Philips, which combines advanced imaging, full solution elastography and precision biopsy.
The solution, designed for diagnosis, screening and follow-up in the case of suspected lesions, is available on EPIQ and Affiniti ultrasound systems. Recently, the monitor size has also been increased to 24 inches with the HD MAX monitor, said Radjen Ganpat, marketing manager for Ultrasound at Philips.
Current customers can upgrade to the latest enhancements.
Qlarity Imaging
Qlarity Imaging is moving its QuantX AI-based decision support software, for helping radiologists in the assessment and characterization of breast abnormalities during breast MR exams, from a traditional dedicated workstation to a vendor-neutral, cloud-based format. This will allow radiologists to use their own viewer and leverage their existing PACS environment, access the secure application anywhere and automatically export their findings into the reporting software.
“We reimagined what breast radiologists need from their technology tools,” said Jon T. DeVries, the chief executive officer of Qlarity Imaging. “With this new format, our goal is to address many of the current challenges health systems and radiologists face and encourage scalable technology that strengthens our vision of transforming the patient journey, including new developments in screening procedures.”
The company plans to submit the new release to the FDA this month and is aiming for clearance in the fourth quarter of 2021.
QT Imaging
QT Imaging has developed a technology called Quantitative Transmission (QT) ultrasound, which uses transmitted sound waves to create three dimensional images of the breast. The technology is different from reflection ultrasound, using 2048 cameras and a water bath to image the breast without compression.
The technology is currently used for both regular and adjunctive screening, and the company is looking to eventually provide an alternative to screening mammography, said John Klock, chief executive officer and chief medical officer of QT Imaging.
A blinded clinical trial comparing the technology to mammography was published in Academic Radiology online in December 2020. The study found that QT scan provided better sensitivity and specificity than mammography, resulting in fewer callbacks.
“We're very happy that women are getting good results from it,” Klock said. “We're hoping women will be our allies.”
The company plans to expand the indications for use and expects to submit for FDA clearance to measure fibroglandular volume in 2021, which can be affected by a woman’s menstrual cycle or hormone therapy.
“For women on hormone therapy to prevent breast cancer, this will allow measurement of how they’re responding,” Klock said.
Siemens Healthineers
Siemens Healthineers has not debuted any new women's health systems or software within the last year. However, the company has been strategizing with its customers to help them cope with the sudden influx of screening examinations following the screening mammography standstill brought on by the pandemic.
For example, the latest software update on MAMMOMAT Revelation has decreased room time, providing a four-view tomosynthesis exam in under six minutes, allowing facilities to provide 10-minute exam slots.
“Our customers were really valuing that coming out of the pandemic,” said Abigail Weldon, director of women’s health at Siemens Healthineers North America.
Automated breast density reading helps radiologists prioritize further diagnostic needs right away.
The company also offers remote installation, training and support.
Volpara
In January, Volpara Health acquired CRA Health, which has an expertise in breast cancer risk and genetics. This best-of-breed breast cancer risk assessment solution was integrated into Volpara’s Patient Hub mammography reporting and patient communication software. The new solution also provides content to help radiologists explain the patient’s breast cancer risk alongside appropriate recommendations, including whether additional imaging or genetics testing is needed according to established guidelines.
“It’s all about personalizing breast care and helping save families from cancer by preventing advanced stage breast cancer,” said Ralph Highnam, chief executive officer of Volpara Health.
Last fall, the company also launched Project Thumb, a new tool that will allow clinicians to embed mammogram images on patients' breast density notification letters with the goal to educate patients to help them make informed decisions about their breast care.
Later this year, the company plans to upgrade its Volpara Analytics platform, which enables technologists to log in and learn about their own acquisition performance and access education on quality imaging techniques.