August's New Product Showcase
August 28, 2014
by Sean Ruck, Contributing Editor
Proteus ONE compact proton therapy system
IBA has received Marketing Authorization from the U.S. Food and Drug Administration for its compact gantry beam line. The company anticipates that this regulatory approval will intensify the international interest in Proteus ONE, its next generation proton therapy compact system.
Proteus ONE is IBA's single-room proton therapy system which is smaller, less expensive, faster to install and encompasses the latest in targeted proton therapy technologies, including IBA's Intensity Modulated Proton Therapy. IBA created the Proteus ONE system to allow more patients access to proton therapy globally and has already sold five Proteus ONE systems in Shreveport, Louisiana (USA), Nice (France), Taiwan (China) and two in Japan.
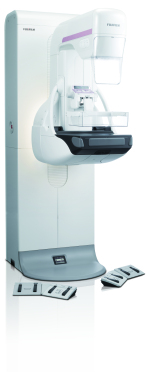
AspireCristalle mammography system
Fujifilm Medical Systems U.S.A., Inc. announced FDA 510(k) clearance of its digital mammography system - Aspire Cristalle.
One of the new features that were created to make the patient more comfortable is the Comfort Paddle. It has soft edges, a flexible composition and four-way pivot that forms to the shape of each individual woman's breast.
It also has patient grip handles and padding, soft backlighting and mural decals in order to try to put the patients at ease during the exam and make it more accessible for women with wheelchairs.
The new Hexagonal Close Pattern is a detector pixel design that provides higher acquisition efficiency in order to get better detail, higher DQE, higher MTF and lower dose compared to traditional square pixel arrays.
The system's image quality was also improved with iAEC automated exposure controls and ISC tuned contrast.
The Real Eye Nano
Avotec's Real Eye Nano integrates a fiber optic illuminator and image guide into the mirror that the patient uses to view the projection screen.
Designed for Nova 32 style head coils where space is at a premium for visual accessories, the system uses IR Illumination (980nm) to illuminate the patient's eye(s). An image is then relayed back to the control room, where it can be viewed and directed to an optional video based Eye Tracker to measure eye movement.
Optional video based Eye Tracking software packages are available.
ClearSight system NONINVASIVE MONITORING SYSTEM
Edwards Lifesciences Corporation announced it has received U.S. Food and Drug Administration (FDA) clearance for the ClearSight system, a noninvasive monitor.
Using a cuff on the outside of the finger that is connected to the Edwards EV1000 clinical platform, the ClearSight system automatically provides up-to-the-minute information without inserting anything into the body and provides an opportunity to extend the benefits of hemodynamic optimization, or proper fluid administration and balance within a patient's organs and tissues, to a broader patient population that could benefit from close monitoring, but may not receive it without a noninvasive option.